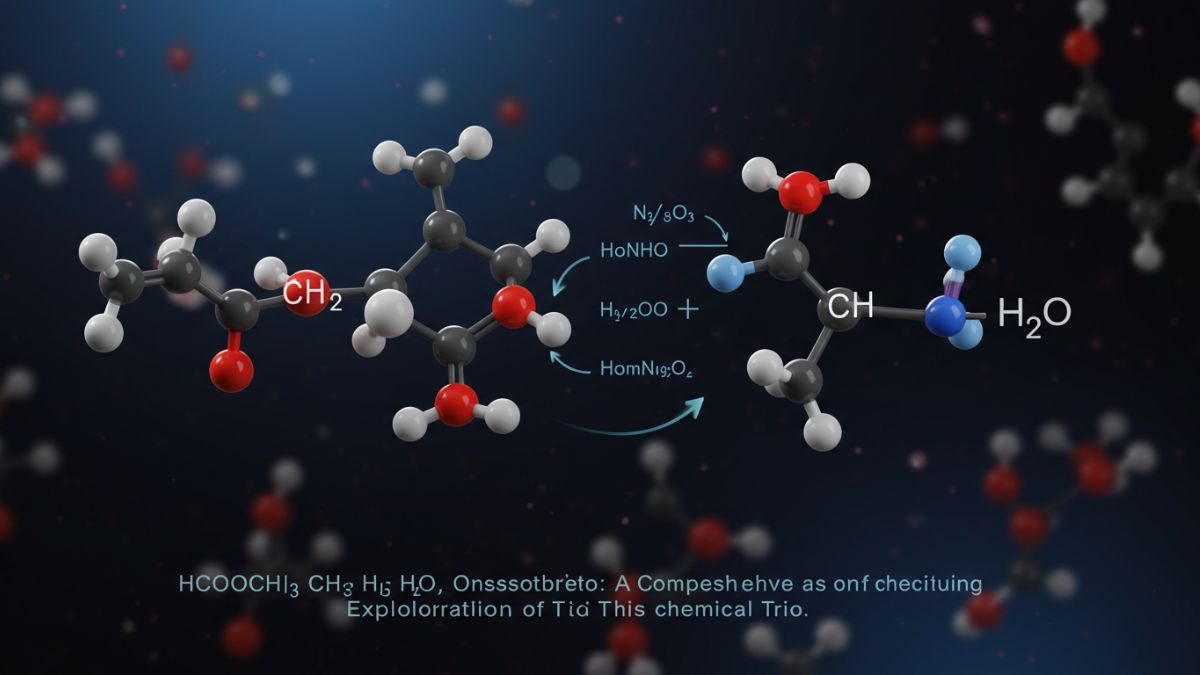
Understanding the nature of chemical compounds and how they interact is central to the study of chemistry. One intriguing combination of compounds that often appears in organic reaction scenarios is HCOOCH CH2 H2O. While it may look like a cryptic formula to some, it actually represents a set of crucial building blocks used in chemical reactions, industrial production, and research.
In this article, we’ll delve deep into what HCOOCH, CH₂, and H₂O signify, their roles in various reactions, and why their combination is significant in the scientific world.
Introduction to HCOOCH CH2 H2O
The term HCOOCH CH2 H2O brings together three distinct molecular components:
-
HCOOCH: This is methyl formate, an ester formed from formic acid and methanol. It is a colorless, flammable liquid with a pleasant odor and is used extensively in organic synthesis.
-
CH₂: This represents a methylene group, a highly reactive part of many organic molecules. It often serves as a bridge or link in carbon chains.
-
H₂O: Water, a universal solvent, often plays a major role in hydrolysis and other chemical reactions.
Together, HCOOCH CH2 H2Os points to possible ester hydrolysis or organic transformation reactions involving water as a reactant.
The Chemistry Behind HCOOCH CH2 H2O
To better grasp the interaction between HCOOCH CH2 H2O, let’s first consider the properties of each.
-
Methyl formate (HCOOCH) is a simple ester. Esters are known for undergoing hydrolysis in the presence of water, especially when an acid or base is present as a catalyst.
-
Methylene (CH₂) groups are key to extending carbon chains and enabling addition reactions.
-
Water (H₂O) acts as a reactant that breaks bonds in esters, converting them back to their original acid and alcohol components.
A basic hydrolysis reaction of methyl formate with water can be written as:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
While the formula CH₂ is not always a direct reactant, it’s often part of reaction intermediates or polymer chains, making HCOOCH CH2 H2Os a potential representation of more complex organic processes.
Applications of the HCOOCH CH2 H2O Reaction
1. Organic Synthesis
The combination of HCOOCH CH2 H2O is vital in creating intermediate products in organic synthesis. Hydrolysis of methyl formate leads to formic acid and methanol—two chemicals widely used in manufacturing, agriculture, and pharmaceuticals.
2. Polymer Chemistry
In certain advanced reactions, the CH₂ group acts as a repeating unit for polymers. When combined with esters like HCOOCH and hydrolysis agents like water, chemists can initiate polyester formation. These reactions are key in producing plastics, fibers, and resins.
3. Green Chemistry
The hydrolysis of HCOOCH CH2 H2Os in water aligns with sustainable and eco-friendly principles. Since no toxic solvents or harsh reagents are needed, it supports the idea of environmentally responsible chemistry.
Reaction Mechanism Involving HCOOCH CH2 H2O
Let’s break down a typical reaction involving these compounds:
Step-by-Step Hydrolysis of Methyl Formate:
-
Protonation of the Ester: Under acidic conditions, the carbonyl oxygen of HCOOCH becomes protonated, increasing its reactivity.
-
Nucleophilic Attack by Water (H₂O): Water attacks the carbon of the carbonyl group.
-
Formation of a Tetrahedral Intermediate: This intermediate rearranges to release methanol (CH₃OH).
-
Deprotonation and Formation of Formic Acid: Final step leads to the creation of formic acid (HCOOH).
The CH₂ group, if part of a larger structure or substrate, may initiate additional chain reactions or polymerization, especially in complex organic syntheses.
Industrial Significance of HCOOCH CH2 H2Os
Industries that rely on chemical synthesis often use reactions involving HCOOCH CH2 H2O. Let’s examine a few practical examples:
-
Formic acid is a preservative and antibacterial agent used in livestock feed.
-
Methanol is a crucial feedstock for producing formaldehyde, plastics, and synthetic fibers.
-
Polymers with CH₂ units are foundational in material science and plastics manufacturing.
Thus, the reaction and chemistry of HCOOCH CH2 H2Os contribute significantly to modern industry.
Laboratory Use and Experimental Setup
In educational and research laboratories, hydrolysis of esters like HCOOCH with H₂O is a common experiment. When conducted with appropriate catalysts and temperature control, it offers insights into reaction kinetics, molecular behavior, and energy changes.
Basic tools used include:
-
Beakers and flasks
-
pH indicators
-
Reflux condenser
-
Titration equipment
The role of CH₂ may be analyzed using spectroscopic methods like IR and NMR, especially if it’s part of a more complex compound undergoing transformation.
Safety Considerations for HCOOCH CH2 H2O
While the compounds discussed are widely used, safety cannot be ignored:
-
HCOOCH (methyl formate) is flammable and should be handled in a well-ventilated area.
-
Methanol, a by-product, is highly toxic and can cause blindness or death if ingested.
-
Water (H₂O) is harmless but must be pure to avoid contamination in reactions.
Always wear gloves, goggles, and lab coats when dealing with chemical reactions involving HCOOCH CH2 H2Os.
Future of Research Involving HCOOCH CH2 H2O
The realm of chemical engineering and molecular design continues to evolve. Scientists are exploring catalyst-free reactions, biological enzymes, and AI-driven predictive modeling to optimize reactions involving compounds like HCOOCH CH2 H2O.
There’s also growing interest in using green solvents and recyclable materials, where these compounds play a key role in developing environmentally friendly chemical processes.
Final Thoughts on HCOOCH CH2 H2O
While the formula HCOOCH CH2 H2O might initially appear complex, it represents a highly significant set of molecules that form the foundation of essential chemical reactions. From hydrolysis to polymer formation and sustainable industrial applications, this chemical trio plays a crucial part in both academic and industrial chemistry.
Whether you’re a student, researcher, or someone curious about how chemistry shapes our world, understanding the behavior and importance of HCOOCH CH2 H2Os opens the door to a deeper appreciation of organic processes.